Product Name :
Methyl Lysine monoclonal antibody Background :
Methylation of lysine residues is a common regulatory post-translational modification (PTM) that results in the mono-, di-, or tri-methylation of lysine at ε-amine groups by protein lysine methyltransferases (PKMTs). Two PKMT groups are recognized based on structure and catalytic mechanism: class I methyltransferases or seven β strand enzymes, and SET domain-containing class V methyltransferases. Both use the methyl donor S-adenosyl-L-methionine to methylate histone and non-histone proteins. Class I methyltransferases methylate amino acids, DNA, and RNA. Six methyl-lysine-interacting protein families are distinguished based on binding domains: MBT, PHD finger, Tudor, PWWP, WD40 repeat, and chromodomains. Many of these display differential binding preferences based on lysine methylation state. KDM1 subfamily lysine demethylases catalyze demethylation of mono- and di-methyl lysines, while 2-oxoglutarate-dependent JmjC (KDM2-7) subfamily enzymes also modify tri-methyl lysine residues.Most PKMT substrates are histone proteins and transcription factors, emphasizing the importance of lysine methylation in regulating chromatin structure and gene expression. Lys9 of histone H3 is mono- or di-methylated by G9A/GLP and tri-methylated by SETDB1 to activate transcription. JHDM3A-mediated demethylation of the same residue creates mono-methyl Lys9 and inhibits gene transcription. Tumor suppressor p53 is regulated by methylation of at least four sites. p53-mediated transcription is repressed following mono-methylation of p53 at Lys370 by SMYD2; di-methylation at the same residue further inhibits p53 by preventing association with 53BP1. Concomitant di-methylation at Lys382 inhibits p53 ubiquitination following DNA damage. Mono-methylation at Lys382 by SET8 suppresses p53 transcriptional activity, while SET7/9 mono-methylation at Lys372 inhibits SMYD2 methylation at Lys370 and stabilizes the p53 protein. Di-methylation at Lys373 by G9A/GLP inhibits p53-mediated apoptosis and correlates with tri-methylation of histone H3 Lys9 at the p21 promoter (1,6). Overexpression of PKMTs is associated with multiple forms of human cancer, which has generated tremendous interest in targeting protein lysine methyltransferases in drug discovery research. Product :
Liquid in 0.42% Potassium phosphate, 0.87% Sodium chloride, pH 7.3, 30% glycerol, and 0.01% sodium azide. Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Specificity :
Recognizes endogenous levels of Pan Methyl Lysine protein. Immunogen :
Purified protein corresponding to Methyl Lysine. Conjugate :
Unconjugated Modification :
Unmodification
Methyl Lysine monoclonal antibody Background :
Methylation of lysine residues is a common regulatory post-translational modification (PTM) that results in the mono-, di-, or tri-methylation of lysine at ε-amine groups by protein lysine methyltransferases (PKMTs). Two PKMT groups are recognized based on structure and catalytic mechanism: class I methyltransferases or seven β strand enzymes, and SET domain-containing class V methyltransferases. Both use the methyl donor S-adenosyl-L-methionine to methylate histone and non-histone proteins. Class I methyltransferases methylate amino acids, DNA, and RNA. Six methyl-lysine-interacting protein families are distinguished based on binding domains: MBT, PHD finger, Tudor, PWWP, WD40 repeat, and chromodomains. Many of these display differential binding preferences based on lysine methylation state. KDM1 subfamily lysine demethylases catalyze demethylation of mono- and di-methyl lysines, while 2-oxoglutarate-dependent JmjC (KDM2-7) subfamily enzymes also modify tri-methyl lysine residues.Most PKMT substrates are histone proteins and transcription factors, emphasizing the importance of lysine methylation in regulating chromatin structure and gene expression. Lys9 of histone H3 is mono- or di-methylated by G9A/GLP and tri-methylated by SETDB1 to activate transcription. JHDM3A-mediated demethylation of the same residue creates mono-methyl Lys9 and inhibits gene transcription. Tumor suppressor p53 is regulated by methylation of at least four sites. p53-mediated transcription is repressed following mono-methylation of p53 at Lys370 by SMYD2; di-methylation at the same residue further inhibits p53 by preventing association with 53BP1. Concomitant di-methylation at Lys382 inhibits p53 ubiquitination following DNA damage. Mono-methylation at Lys382 by SET8 suppresses p53 transcriptional activity, while SET7/9 mono-methylation at Lys372 inhibits SMYD2 methylation at Lys370 and stabilizes the p53 protein. Di-methylation at Lys373 by G9A/GLP inhibits p53-mediated apoptosis and correlates with tri-methylation of histone H3 Lys9 at the p21 promoter (1,6). Overexpression of PKMTs is associated with multiple forms of human cancer, which has generated tremendous interest in targeting protein lysine methyltransferases in drug discovery research. Product :
Liquid in 0.42% Potassium phosphate, 0.87% Sodium chloride, pH 7.3, 30% glycerol, and 0.01% sodium azide. Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Specificity :
Recognizes endogenous levels of Pan Methyl Lysine protein. Immunogen :
Purified protein corresponding to Methyl Lysine. Conjugate :
Unconjugated Modification :
Unmodification
-
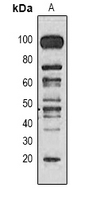 Western blot analysis of Methyl Lysine expression in Hela (A) whole cell lysates.
Western blot analysis of Methyl Lysine expression in Hela (A) whole cell lysates. -
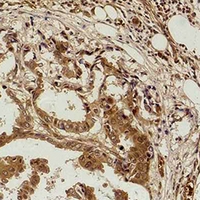 Immunohistochemical analysis of Methyl Lysine staining in human breast cancer formalin fixed paraffin embedded tissue section. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH 6.0). The section was then incubated with the antibody at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX.
Immunohistochemical analysis of Methyl Lysine staining in human breast cancer formalin fixed paraffin embedded tissue section. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH 6.0). The section was then incubated with the antibody at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX.
Bioworld Biotech only provide peptides for our antibodies and do not provide additional peptide customization services.
Price/Size :
USD 368/1mg/vial
Tips:
For phospho antibody, we provide phospho peptide(0.5mg) and non-phospho peptide(0.5mg).Describe :
Blocking peptides are peptides that bind specifically to the target antibody and block antibody binding. These peptide usually contains the epitope recognized by the antibody. Antibodies bound to the blocking peptide no longer bind to the epitope on the target protein. This mechanism is useful when non-specific binding is an issue, for example, in Western blotting (WB) and Immunohistochemistry (IHC). By comparing the staining from the blocked antibody versus the antibody alone, one can see which staining is specific; Specific binding will be absent from the western blot or IHC performed with the neutralized antibody.Formula:
Synthetic peptide was lyophilized with 100% acetonitrile and is supplied as a powder. Reconstitute with 0.1 ml DI water for a final concentration of 10 mg/ml.The purity is >90%,tested by HPLC and MS.
Storage:
The freeze-dried powder is more stable. For short time at 2-8°C. For long term storage store at -20°C.
Note :
This product is for research use only (RUO only). Not for use in diagnostic or therapeutic procedures.
 Methyl Lysine monoclonal antibody
Methyl Lysine monoclonal antibody  Datasheet
Datasheet COA
COA MSDS
MSDS SHIP
SHIP