Product Name :
Histone H2B (Butyryl-K20) polyclonal antibody Background :
The nucleosome, made up of four core histone proteins (H2A, H2B, H3, and H4), is the primary building block of chromatin. Originally thought to function as a static scaffold for DNA packaging, histones have now been shown to be dynamic proteins, undergoing multiple types of post-translational modifications, including acetylation, phosphorylation, methylation, and ubiquitination . The p300/CBP histone acetyltransferases acetylate multiple lysine residues in the amino terminal tail of histone H2B (Lys5, 12, 15, and 20) at gene promoters during transcriptional activation . Hyper-acetylation of the histone tails neutralizes the positive charge of these domains and is believed to weaken histone-DNA and nucleosome-nucleosome interactions, thereby destabilizing chromatin structure and increasing the access of DNA to various DNA-binding proteins . In addition, acetylation of specific lysine residues creates docking sites that facilitate recruitment of many transcription and chromatin regulatory proteins that contain a bromodomain, which binds to acetylated lysine residues . Histone H2B is mono-ubiquitinated at Lys120 during transcriptional activation by the RAD6 E2 protein in conjunction with the BRE1A/BRE1B E3 ligase (also known as RNF20/RNF40). Mono-ubiquitinated histone H2B Lys120 is associated with the transcribed region of active genes and stimulates transcriptional elongation by facilitating FACT-dependent chromatin remodeling . In addition, it is essential for subsequent methylation of histone H3 Lys4 and Lys79, two additional histone modifications that regulate transcriptional initiation and elongation . In response to metabolic stress, AMPK is recruited to responsive genes and phosphorylates histone H2B at Lys36, both at promoters and in transcribed regions of genes, and may regulate transcriptional elongation . In response to multiple apoptotic stimuli, histone H2B is phosphorylated at Ser14 by the Mst1 kinase . Upon induction of apoptosis, Mst1 is cleaved and activated by caspase-3, leading to global phosphorylation of histone H2B during chromatin condensation. Interestingly, histone H2B is rapidly phosphorylated at irradiation-induced DNA damage foci in mouse embryonic fibroblasts . In this case, phosphorylation at Ser14 is rapid, depends on prior phosphorylation of H2AX Ser139, and occurs in the absence of apoptosis, suggesting that Ser14 phosphorylation may have distinct roles in DNA-damage repair and apoptosis. Product :
Liquid in 0.42% Potassium phosphate, 0.87% Sodium chloride, pH 7.3, 30% glycerol, and 0.01% sodium azide. Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Specificity :
Recognizes endogenous levels of Histone H2B with a site at Butyryl-K20 protein. Immunogen :
KLH-conjugated synthetic peptide encompassing a sequence within the N-term region of human Histone H2B with a site at Butyryl-K20. The exact sequence is proprietary. Conjugate :
Unconjugated Modification :
Unmodification
Histone H2B (Butyryl-K20) polyclonal antibody Background :
The nucleosome, made up of four core histone proteins (H2A, H2B, H3, and H4), is the primary building block of chromatin. Originally thought to function as a static scaffold for DNA packaging, histones have now been shown to be dynamic proteins, undergoing multiple types of post-translational modifications, including acetylation, phosphorylation, methylation, and ubiquitination . The p300/CBP histone acetyltransferases acetylate multiple lysine residues in the amino terminal tail of histone H2B (Lys5, 12, 15, and 20) at gene promoters during transcriptional activation . Hyper-acetylation of the histone tails neutralizes the positive charge of these domains and is believed to weaken histone-DNA and nucleosome-nucleosome interactions, thereby destabilizing chromatin structure and increasing the access of DNA to various DNA-binding proteins . In addition, acetylation of specific lysine residues creates docking sites that facilitate recruitment of many transcription and chromatin regulatory proteins that contain a bromodomain, which binds to acetylated lysine residues . Histone H2B is mono-ubiquitinated at Lys120 during transcriptional activation by the RAD6 E2 protein in conjunction with the BRE1A/BRE1B E3 ligase (also known as RNF20/RNF40). Mono-ubiquitinated histone H2B Lys120 is associated with the transcribed region of active genes and stimulates transcriptional elongation by facilitating FACT-dependent chromatin remodeling . In addition, it is essential for subsequent methylation of histone H3 Lys4 and Lys79, two additional histone modifications that regulate transcriptional initiation and elongation . In response to metabolic stress, AMPK is recruited to responsive genes and phosphorylates histone H2B at Lys36, both at promoters and in transcribed regions of genes, and may regulate transcriptional elongation . In response to multiple apoptotic stimuli, histone H2B is phosphorylated at Ser14 by the Mst1 kinase . Upon induction of apoptosis, Mst1 is cleaved and activated by caspase-3, leading to global phosphorylation of histone H2B during chromatin condensation. Interestingly, histone H2B is rapidly phosphorylated at irradiation-induced DNA damage foci in mouse embryonic fibroblasts . In this case, phosphorylation at Ser14 is rapid, depends on prior phosphorylation of H2AX Ser139, and occurs in the absence of apoptosis, suggesting that Ser14 phosphorylation may have distinct roles in DNA-damage repair and apoptosis. Product :
Liquid in 0.42% Potassium phosphate, 0.87% Sodium chloride, pH 7.3, 30% glycerol, and 0.01% sodium azide. Storage&Stability :
Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze-thaw cycles. Specificity :
Recognizes endogenous levels of Histone H2B with a site at Butyryl-K20 protein. Immunogen :
KLH-conjugated synthetic peptide encompassing a sequence within the N-term region of human Histone H2B with a site at Butyryl-K20. The exact sequence is proprietary. Conjugate :
Unconjugated Modification :
Unmodification
-
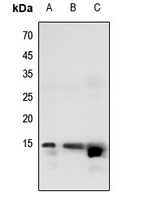 Western blot analysis of Histone H2B (Butyryl-K20) expression in HEK293T (A), U2OS (B), DLD (C) whole cell lysates.
Western blot analysis of Histone H2B (Butyryl-K20) expression in HEK293T (A), U2OS (B), DLD (C) whole cell lysates. -
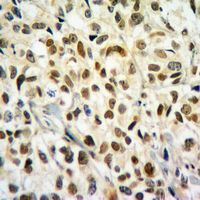 Immunohistochemical analysis of Histone H2B (Butyryl-K20) staining in human breast cancer formalin fixed paraffin embedded tissue section. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH 6.0). The section was then incubated with the antibody at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX.
Immunohistochemical analysis of Histone H2B (Butyryl-K20) staining in human breast cancer formalin fixed paraffin embedded tissue section. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH 6.0). The section was then incubated with the antibody at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX.
Bioworld Biotech only provide peptides for our antibodies and do not provide additional peptide customization services.
Price/Size :
USD 368/1mg/vial
Tips:
For phospho antibody, we provide phospho peptide(0.5mg) and non-phospho peptide(0.5mg).Describe :
Blocking peptides are peptides that bind specifically to the target antibody and block antibody binding. These peptide usually contains the epitope recognized by the antibody. Antibodies bound to the blocking peptide no longer bind to the epitope on the target protein. This mechanism is useful when non-specific binding is an issue, for example, in Western blotting (WB) and Immunohistochemistry (IHC). By comparing the staining from the blocked antibody versus the antibody alone, one can see which staining is specific; Specific binding will be absent from the western blot or IHC performed with the neutralized antibody.Formula:
Synthetic peptide was lyophilized with 100% acetonitrile and is supplied as a powder. Reconstitute with 0.1 ml DI water for a final concentration of 10 mg/ml.The purity is >90%,tested by HPLC and MS.
Storage:
The freeze-dried powder is more stable. For short time at 2-8°C. For long term storage store at -20°C.
Note :
This product is for research use only (RUO only). Not for use in diagnostic or therapeutic procedures.
 Histone H2B (Butyryl-K20) polyclonal antibody
Histone H2B (Butyryl-K20) polyclonal antibody  Datasheet
Datasheet COA
COA MSDS
MSDS SHIP
SHIP